SMA Mineral - A world full of lime
Our basic minerals, limestone and dolomite, can be further processed through burning or slaking. These processed products acquire different physical and chemical properties and entirely new applications.
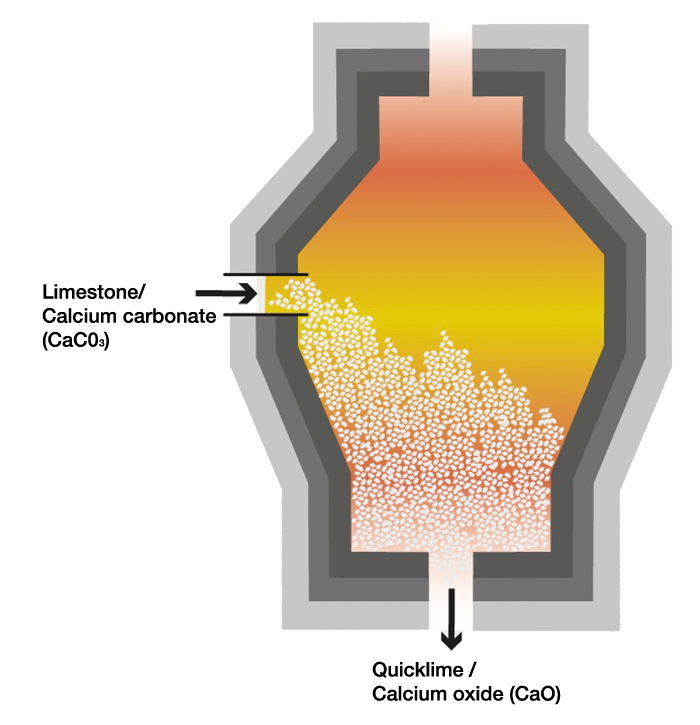





Quicklime
Slaked Lime
Burnt Dolomite
Mesa
Mesa can be recovered by re-burning it into burnt lime and then reusing it in the pulp mills.

SMA Mineral AB
Box 329
682 27 Filipstad
SWEDEN
